On Feb 7th, 2020, the type testing reports of 2019 Novel Coronavirus Antigen Detection Reagent (Immunochromatographic Method) and 2019 Novel Coronavirus Antibody Detection Reagent (Immunochromatographic Method) newly developed by Guangzhou Wondfo Biotech Co.,Ltd. (Stock Code: 300482) successfully issued by Guangdong Province Medical Devices Quality Surveillance and Test Institute, and it was entitled to further process.
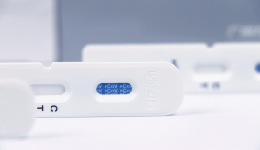
2019 Novel Coronavirus Antigen Detection Reagent (Immunochromatography) can be used for the qualitative detection of coronavirus 2019-nCoV antigen in throat swabs and lower respiratory tract samples. It can help the diagnosis of novel coronavirus,providing evidence-based approaches for healthcare professionals to identify the infected patients, so that the proper precautions and restrictions can be implemented as soon as possible for better controlling the spreading of the virus.
2019 Novel Coronavirus Antibody Detection Reagent (Immunochromatography)can be used for the qualitative detection of 2019-nCoV antibodies in human serum, plasma, and whole blood samples. This rapid test is good for the first step screening of the suspected infected patients.


We strive for excellence to safeguard everyone’s health, and fight against the epidemic!
 The most developed technology platform with a variety of applications, including infectious disease detection, fertility detection, the drug of abuse detection, etc.
The most developed technology platform with a variety of applications, including infectious disease detection, fertility detection, the drug of abuse detection, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.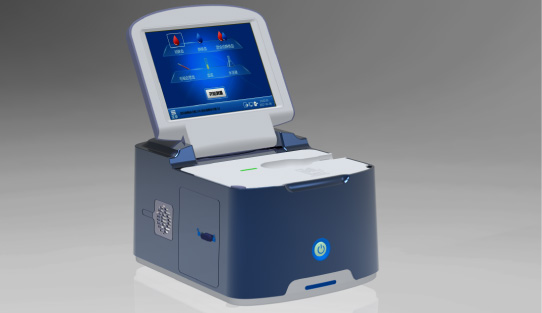 Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.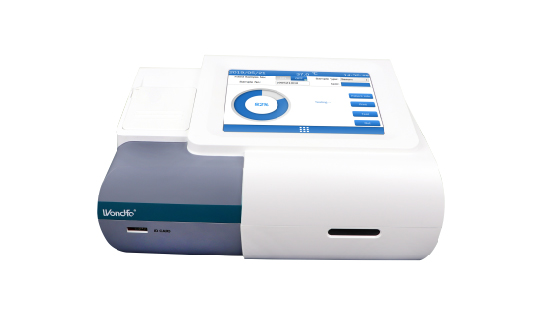 Wondfo Dry Chemistry Analyzer can test 16 items and provide the result within 2 minutes, ideal for the detection of cardiac markers, kidney function, liver function, pancreatitis, etc.
Wondfo Dry Chemistry Analyzer can test 16 items and provide the result within 2 minutes, ideal for the detection of cardiac markers, kidney function, liver function, pancreatitis, etc. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation

































