Novel Coronavirus COVID-19 Rapid Tests were recommended by the State Council
February 21,2020 (Edit)
On Feb 19th 2020, two novel coronavirus detection products developed by Guangzhou Wondfo Biotech Co.,Ltd., had the honor to access to emergency approval channel issued by National Medical Products Administration under recommendation of Scientific research team of Joint Prevention and Control Mechanism of the State Council. This opening bidding has 300 applications. Only a total of 9 programs were selected this time, 2 of them are originated from Wondfo.
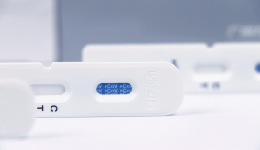
One of listed products developed by Wondfo is novel coronavirus (COVID-19) antigen rapid test kit. It is suitable for conducting a large scale on-site coronavirus screening.
Another product is novel coronavirus (COVID-19) antibody rapid test kit which is based on fluorescence microsphere/colloidal gold immunochromatography technology.
As a leading enterprise of POCT, Wondfo has been dedicating to make good use of our technological advantages to combat against novel coronavirus (COVID-19) epidemic. Clinical verifications and registration of novel coronavirus rapid test kits run smoothly so far, and we will keep attaching great importance to accelerate the process of fighting the epidemic.
From SARS to H1N1、H7N9 outbreak,from Flu to COVID-19 epidemic, Wondfo always fight against the disease in the frontline with the conviction that life is our top priority.
 The most developed technology platform with a variety of applications, including infectious disease detection, fertility detection, the drug of abuse detection, etc.
The most developed technology platform with a variety of applications, including infectious disease detection, fertility detection, the drug of abuse detection, etc. 50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others.
50+ kinds of reagents and five high-performance devices, focusing on detecting cardiovascular disease, inflammation, kidney injury, sex hormones, thyroid function, diabetes, tumor, and others. Single-dose Chemiluminescense Immunoassay Platform
Single-dose Chemiluminescense Immunoassay Platform Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.
Wondfo optical blood coagulation analyzer is the first one in the world that can test PT, APTT, TT, FIB, and ACT simultaneously.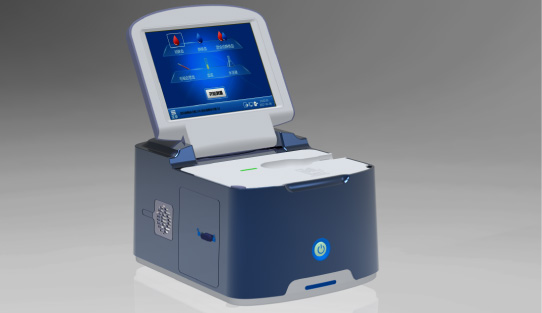 Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.
Our Blood Gas Analyzer BGA-102 can produce the result in 30s. Its advantages of portability, easy operation, durability, and high performance make it ideal for clinics, laboratories, and hospitals.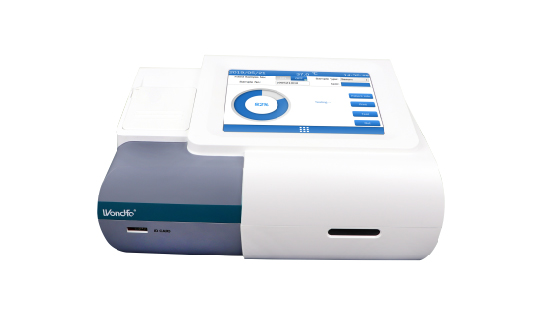 Wondfo Dry Chemistry Analyzer can test 16 items and provide the result within 2 minutes, ideal for the detection of cardiac markers, kidney function, liver function, pancreatitis, etc.
Wondfo Dry Chemistry Analyzer can test 16 items and provide the result within 2 minutes, ideal for the detection of cardiac markers, kidney function, liver function, pancreatitis, etc. Ready-to-use lyophilized RT-PCR Reagent;
Ready-to-use lyophilized RT-PCR Reagent; Wondfo PA-3600 IHC Staining System
Wondfo PA-3600 IHC Staining System The Future Intelligent Medical Assistant to Healthcare
The Future Intelligent Medical Assistant to Healthcare Fight against the pandemic through continuous innovation
Fight against the pandemic through continuous innovation

































